Cyclohexane Reaction With Bromine
C 6 H 12 1R2R-12-dimethylcyclobutane. C 6 H 12 Z-hex-3-ene.

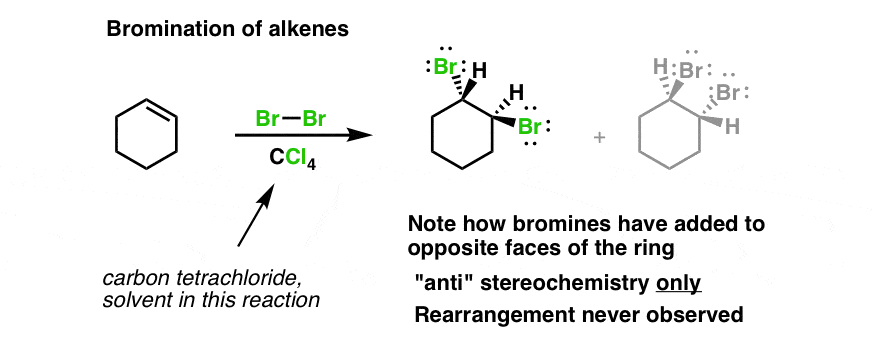
Bromination Of Cyclohexene Under Conditions Given Below Yields Ltimg Src Https D10lpgp6xz60nq Youtube
A demonstration of bromine substitution and addition reactions is helpful at this point.

. The 10 is the use of octanedioic acid sebacic acid instead of hexanedioic acid adipic acid in the reaction with the amine 16-diaminohexane hexamethylenediamine. The reaction involves oxidation of the nitrogen followed by rearrangement of the carbonyl and nitrogen to give an isocyanate intermediate. S N 1 and S N 2.
As suggested by a KIE value of 4 in a parallel experiment with cyclohexane and its fully deuterated analogue. An example is the production of polyamide 610. Then the two identical methyl groups are either cis or trans to each other and the two identical hydrogen atoms are either cis or trans to each other.
Benzene does not react with bromine unless a very bright light or a strong catalyst is used and then the reaction is not an addition reaction. The reaction starts with the base-promoted sulfolene ring-opening to furnish the dienylsulfinate intermediate which transmetalates with the in situ generated aryl. Show how the energy of a chemical reaction can be given out as light by revealing how a solution of sodium chlorateI oxidises an aqueous solution of luminol 3-aminophthalhydrazide to produce a blue chemiluminescent glow without any increase in temperature.
S N 2 examples. The Hofmann rearrangement Hofmann degradation is the organic reaction of a primary amide to a primary amine with one fewer carbon atom. The configuration of an unsaturated carbons include straight chain such as alkenes and alkynes as well as branched.
2 o Benzyl Chloride with HS S N 2 Mechanism. Benzyl Chloride with HS S N 2 Reaction. Bromine and iodine with this demonstration.
What stereoisomers are obtained from the reaction of each of the following alkenes with OsO 4 followed by aqueous H 2 O 2. The reaction between a CC double bond and bromine Br 2 can be used as a test for the presence of alkene in an unknown sample. Includes kit list and safety.
Figure 81b E2 Reaction Mechanism. Achiral compounds with more than one stereogenic centre Meso. The reaction rates were measured by using cyclohexane k H and cyclohexane-d 12 k D as substrates fig.
Stability and structure of carbocations. Purity depends on the amount of C2 and higher hydrocarbons in the methane and the extent of chlorination. Allyl Chloride with HS S N 2 Reaction.
The base OH uses its electron pair to attack a β-hydrogen on β-carbon and starts to form a bond. C 4 H 4 N 2. Simple S N 2 reaction.
When bromine is added to the sample if the reddish color disappears it means the sample contains an alkene. Propylene oxide cyclohexane andor 2-methyl-2-butene are added as stabilizers. Electrophilic addition to alkenes Symmetrical and Unsymmetrical.
This is easy to mistake when hurrying so be careful when you are intepreting any structural formulas which include hexagons. For more than 40 years Flinn has been the Safer Source for Science. The synthetic utility was further demonstrated by the conversion of.
Consisting all single bonds. BrF-bromine fluoride anion. The reaction can form a wide range of products including alkyl and aryl amines.
The E2 mechanism is also a single-step concerted reaction similar to S N 2 with multiple electron pair transfers that happen at the same time. The product is cyclohexane and the heat of reaction provides evidence of benzenes thermodynamic stability. Flinn Scientific is the 1 source for science supplies and equipment both in and outside the classroom.
A simple S N 2 reaction. A KIE value of 112 k H k D was determined from parallel reactions 25 which indicated that the CHCD activation by Cl was not involved in the turnover-limiting step. Substituted benzene rings may also be deduced in this fashion and hydroxy-substituted compounds such as phenol catechol and resorcinol give.
It is simply cyclohexane and there are two hydrogens on each carbon atom. Unsaturated hydrocarbons are hydrocarbons that have double or triple covalent bonds between adjacent carbon atomsThe term unsaturated means more hydrogen atoms may be added to the hydrocarbon to make it saturated ie. Electrophilic Addition Addition of bromine to an alkene.
One such development has been the production of polyamides which are made at least in part from renewable raw materials. The bromine reagent is in a reddish color and the product vicinal dibromide is colorless. The ambiguity comes from the definition of similar groups.
The cis-trans definition is unambiguous only when you have two different groups on one of the alkene carbons and the same two groups on the other carbon as in but-2-ene. Bromine monoxide anion BrO Bromine monoxide cation C v. S N 2 Reaction.
Substrate structure controls substitution mechanism S N 1 or S N 2. At the same time the β C-H sigma bond begins to move in to become the π bond of a double bond and. Methylene Chloride is derived from the chlorination of methane during which other chlorinated methane derivatives may be formed.

Scheme 4 A Reaction Of Cyclohexene With Electrogenerated Bromine In Download Scientific Diagram

Organic Chemistry Product Of Reaction Between Cyclohexene And Bromine In Methanol At 273 K Chemistry Stack Exchange
Bromination Of Alkenes Master Organic Chemistry
Reaction Of Bromine With Cyclohexane Cyclohexene And Benzene
No comments for "Cyclohexane Reaction With Bromine"
Post a Comment